Formation Mechanisms and Control Strategies for Red Scale and Copper Brittle Defects in Hot-Rolled Strip
Red scale and copper brittle defects are common quality issues in hot-rolled strip production, with red scale defects in high-silicon steel grades being a persistent challenge affecting surface quality. Over recent decades, researchers from Japan and other countries have made notable progress in understanding red scale and copper brittleness. This article compiles research methods, key findings, and technical approaches related to these defects for reference.
Keywords: Hot-rolled strip production, red scale, copper brittleness, defect control
The production of steel plate and strip involves high-temperature oxidation, which accounts for approximately one-third of surface quality defects. Among these, red scale and copper brittle (also referred to as hot brittleness) defects are the most prevalent. In 1994, Japanese researcher Fukagawa Chiki et al. proposed the classical mechanism of red scale formation, which has been widely referenced in literature over the past 30 years. Concurrently, studies on the effect of silicon on red scale defects have advanced, forming a relatively mature theoretical system. However, certain practical aspects remain unclear. The author identified such a point during production practice, which was further investigated and validated. Some conclusions align with findings from scholars at China Steel Corporation in Taiwan. Whether red scale defects are solely attributable to subscale formed in the heating furnace—and not fully removed—warrants further examination.
Copper brittle defects are also common in hot-rolled thin strip. This paper introduces Japanese research on the copper embrittlement mechanism and clarifies the rationale behind industry measures to control it. Japanese scholars have conducted intriguing studies in this area, some of which may not yet be suitable for large-scale application, such as certain copper embrittlement suppression methods.
1. Red Scale Defects in Hot-Rolled Strip
1.1 Conventional Theory of Red Scale Formation
In the 1990s, Japanese researchers proposed a formation mechanism for red scale defects in hot-rolled strip, which gained broad acceptance and has been frequently cited.
Tomoki FUKAGAWA et al. investigated the role of silicon using two steel grades: 0.09%C-0.54%Si-1.46%Mn and 0.05%C-0.15%Mn. Slabs were heated at 1220°C for 2 hours in an atmosphere of 77.1% N₂, 14.3% H₂O, and 8.2% CO₂. The formation of primary scale was controlled using a stainless steel cover.
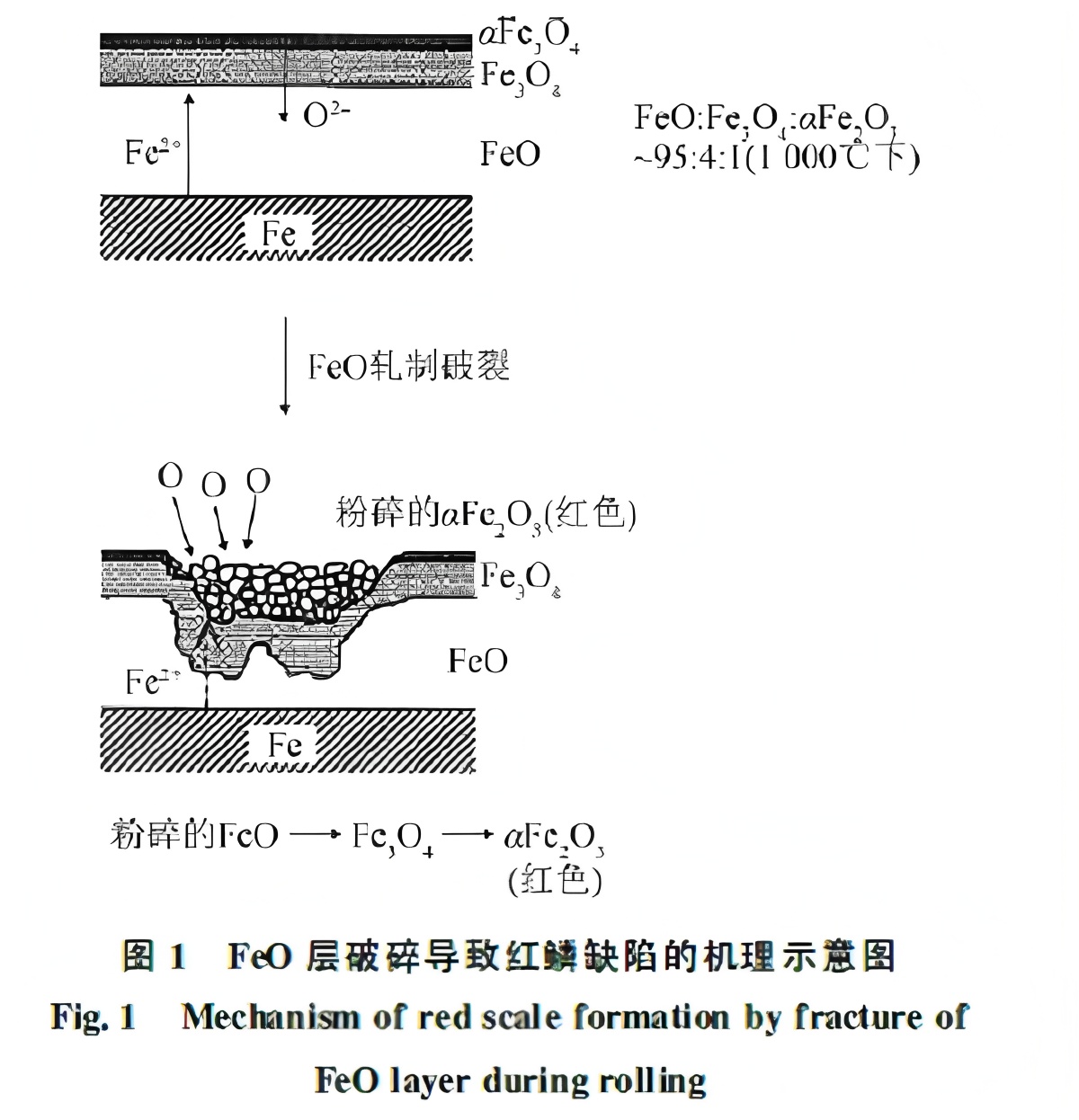
It was found that even in silicon-free steel, incomplete descaling before rolling can lead to red scale formation. When heated at 1000°C in air, the oxides formed include FeO, Fe₃O₄, and Fe₂O₃ in a volume ratio of approximately 95:4:1. If FeO is fractured during rolling, the broken particles may be reduced by matrix Fe²⁺ ions but are also exposed to oxygen, accelerating the conversion to Fe₃O₄ and Fe₂O₃. The formation mechanism is illustrated in Figure 1. Studies indicate that the color intensity of red scale is determined by the proportion of Fe₂O₃ particles smaller than 2 μm.
Figure 2 illustrates the red scale formation mechanism in silicon-containing steel. Since red scale defects occur only on slabs with primary scale formed in the furnace, researchers concluded that these defects are closely related to furnace-grown scale. Further analysis revealed that a eutectic compound of FeO and Fe₂SiO₄ penetrates FeO grains and the steel matrix, forming an “anchor structure” that impedes descaling.
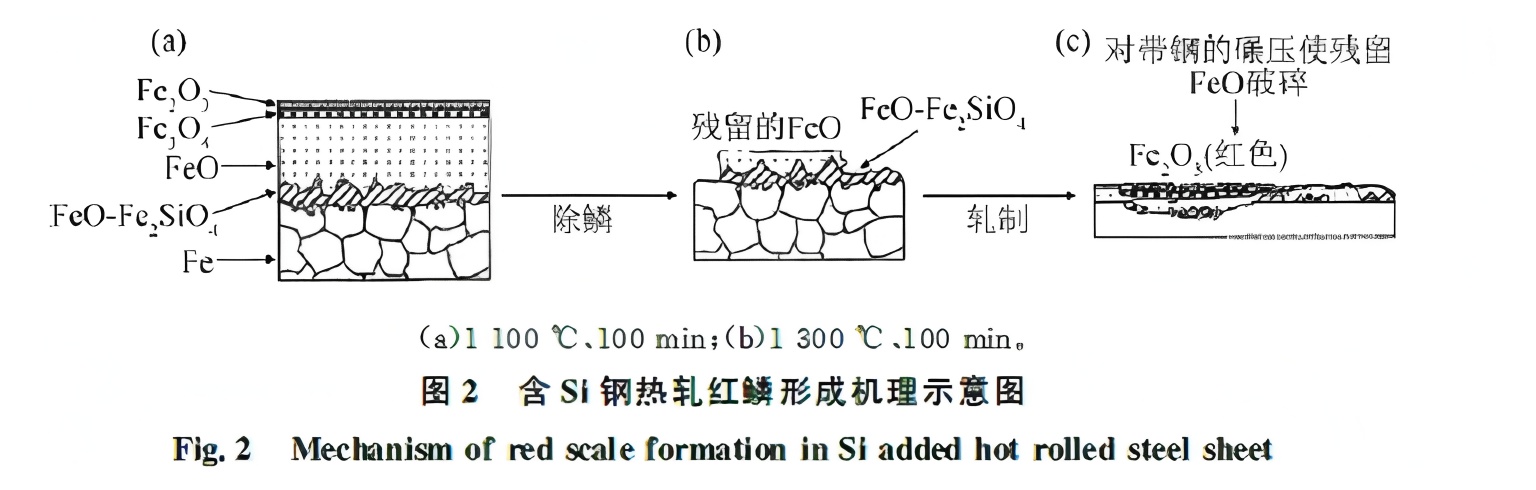
The melting point of Fe₂SiO₄ is 1173°C. At 1220°C, it is liquid and envelops FeO particles, forming a eutectic structure with an anchor-like morphology. This eutectic can undergo plastic deformation just below its solidus temperature (1163°C) but becomes hard and brittle at 1107°C. During rolling, FeO particles fracture and oxidize further to Fe₂O₃. The high-strength eutectic compound is difficult to remove via descaling below 1173°C. Fukagawa et al. support the view that descaling is more effective when the eutectic is liquid.
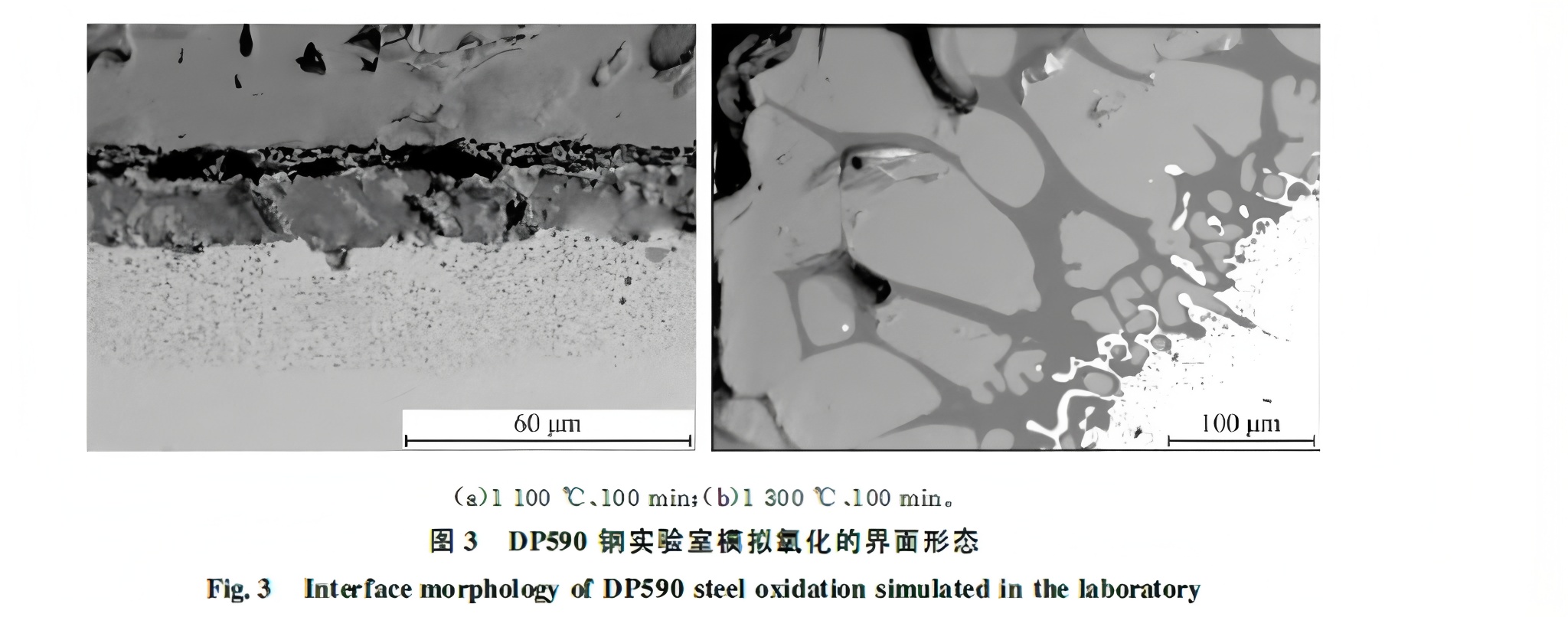
Figure 3 shows the oxidation interface from a differential thermal analysis simulation. The test used DP590 steel (0.45% Si) in a 2% O₂ and balance H₂ atmosphere for 100 minutes. At 1100°C, solid Si-rich particles (SiO₂ + Fe₂SiO₄) form. At 1300°C, Fe₂SiO₄ liquefies, penetrates the iron matrix forming an anchor structure, and spreads along grain boundaries in a network pattern, which is considered responsible for difficult scale removal.
1.2 Factors Influencing Red Scale Defect Formation
1.2.1 Influence of Alloying Elements Si and Ni
Silicon plays the most significant role among alloying elements affecting high-temperature oxidation behavior of steel.
The addition of silicon (0.1% to 1.5% by mass) to industrial pure iron revealed that at 1100°C, a silicon-enriched layer acts protectively, reducing oxidation with increasing Si content. At 1200°C, however, liquid Fe₂SiO₄ forms, increasing oxidation. Notably, for 0.5% Si steel at 1150°C, although the phase diagram suggests Fe₂SiO₄ should not be liquid, oxidation behavior indicates localized liquid phase formation due to overheating from surface reactions, accelerating oxidation.
Tata Steel examined the optimal composition (0.06%C-1.6%Mn-0.15%Si-1%Ni) for achieving desired properties and its effect on oxide formation and descaling. The heating atmosphere simulated actual furnace exhaust gas (8.8% CO₂, 1.7% O₂, 18% H₂O, 71.5% N₂). This study highlighted the role of nickel: the combination of Si and Ni enhances interface entanglement, severely impacting descaling. Ni-rich metal particles distribute near pores in the FeO scale, particularly around Fe₂SiO₄, increasing red scale susceptibility.
1.2.2 Effect of Hot Rolling and Descaling Conditions
Hikaru OKADA and Tomoki FUKAGAWA used a three-stand模拟轧机 to study the effects of hot rolling and descaling on red scale defects in high-silicon steel.
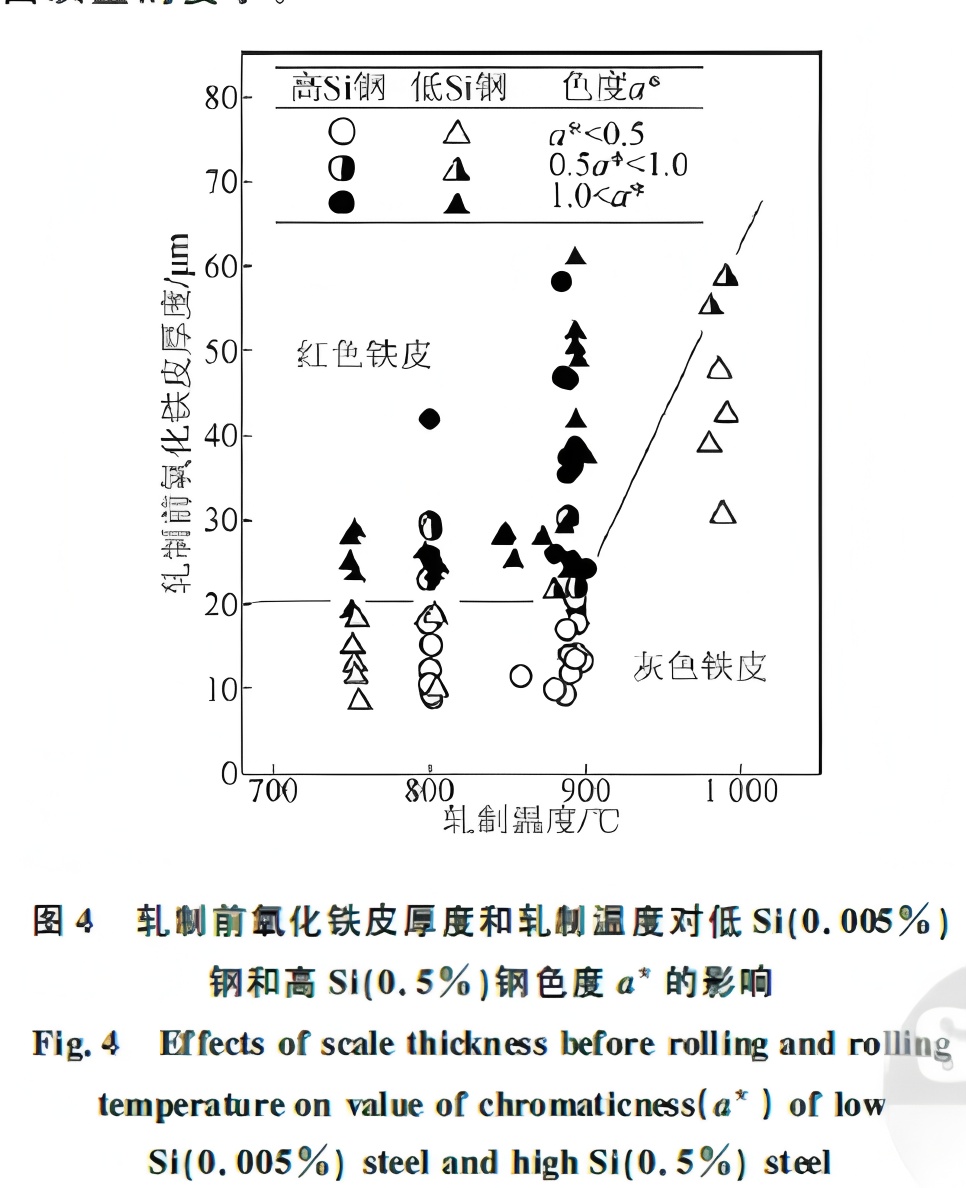
Results showed that red scale forms when the scale thickness before rolling exceeds 20 μm and the rolling temperature is below 900°C, regardless of silicon content. This is because, below 900°C, the outer scale (mainly FeO) pulverizes, and the powder oxidizes during cooling to form red Fe₂O₃. With decreasing rolling temperature, the amount of pulverization increases. While descaling can fully remove thick scale from low-silicon steel, the strong adhesion of Fe₂SiO₄ in high-silicon steel causes residual scale, leading to red scale defects. After descaling, high-silicon slabs retain a 40–180 μm thick FeO layer. The relationship between rolling temperature, scale thickness, and color (chroma a*) is shown in Figure 4.
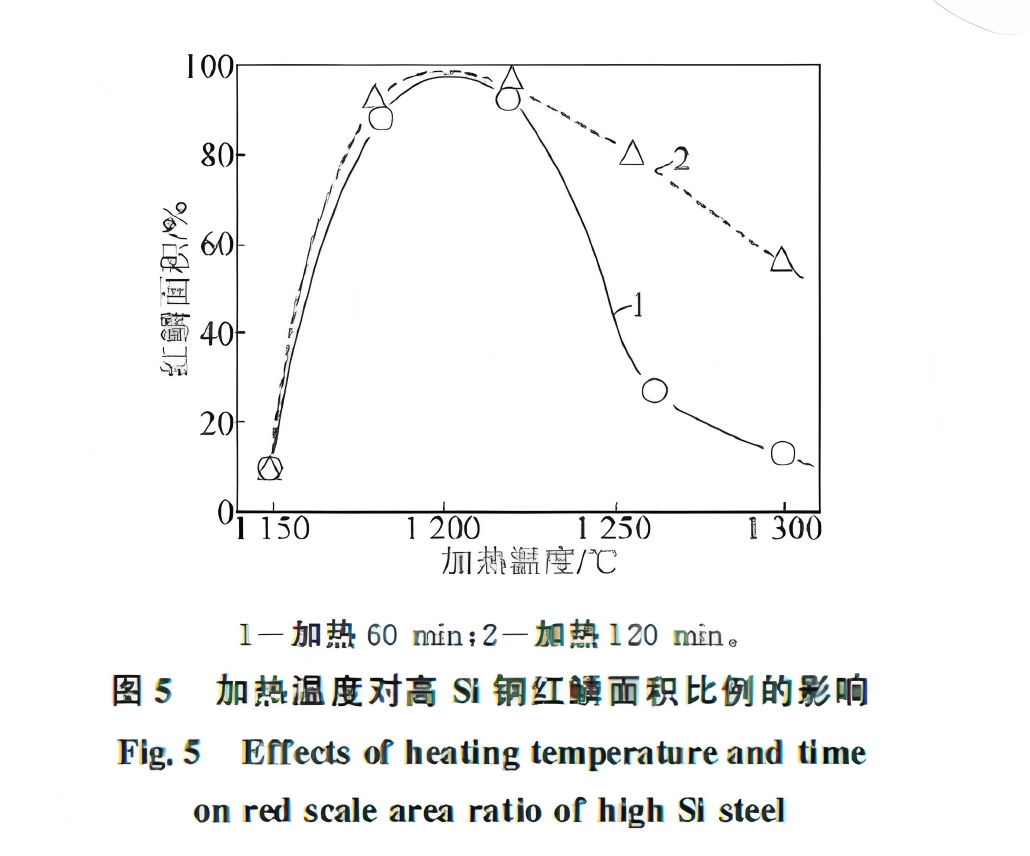
Considering the deformation characteristics of FeO, effective descaling and high-temperature finishing rolling are essential for low-silicon steel. For high-silicon steel, descaling after slab heating is critical and requires higher pressure at temperatures above the Fe₂SiO₄ melting point (1173°C). For high-silicon (e.g., 0.5% Si) steel, using a heating temperature below 1173°C (e.g., 1150°C) can prevent Fe₂SiO₄ liquefaction, or an extremely high temperature (e.g., 1300°C) can ensure the eutectic remains liquid during descaling, reducing red scale. However, the effectiveness of high-temperature heating decreases with prolonged time, as shown in Figure 5: extending heating time at 1300°C from 60 to 120 minutes increases red scale area from 15% to 60%, making it unsuitable for production requiring high surface quality.
Notably, if a slab is heated under a stainless steel cover to prevent primary scale formation, only secondary scale forms, and no red scale occurs regardless of silicon content, because secondary scale forms well below 1173°C and is easily removed.
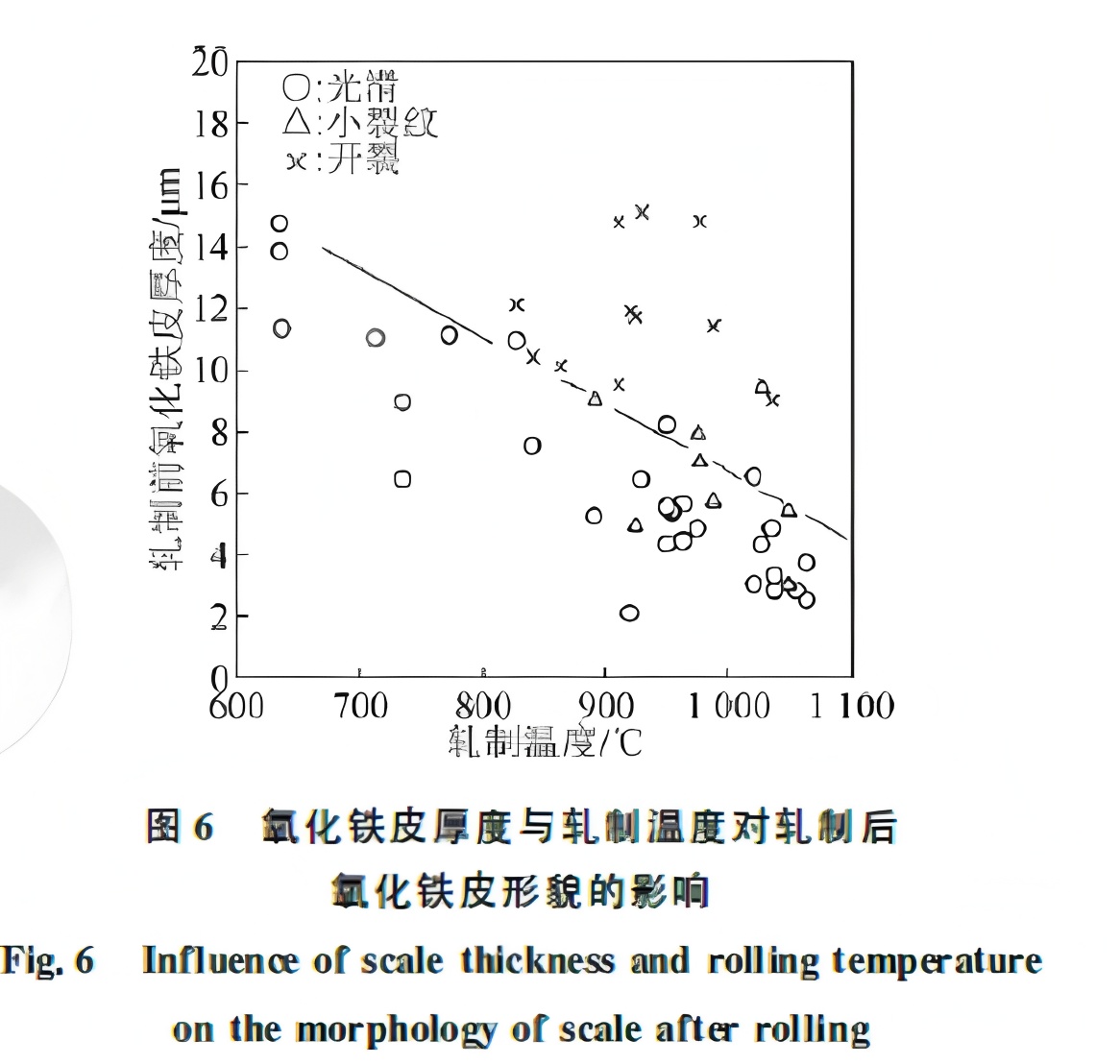
Two decades later (2015), Hikaru Okada further investigated scale deformation and red scale formation during strip rolling. Using low-carbon steel (0.05%C-0.15%Mn), slabs were heated under N₂ or in a stainless steel box to simulate different primary scale thicknesses by varying delay time on the roller table before rolling.
When scale is thin before rolling, the rolled scale is smooth and uniform. However, with pre-rolling scale ≥10 μm, visible cracks form after rolling. Thicker scale and higher rolling temperatures promote such cracking, as shown in Figure 6. Interestingly, the compression deformation of the scale closely matches that of the matrix.
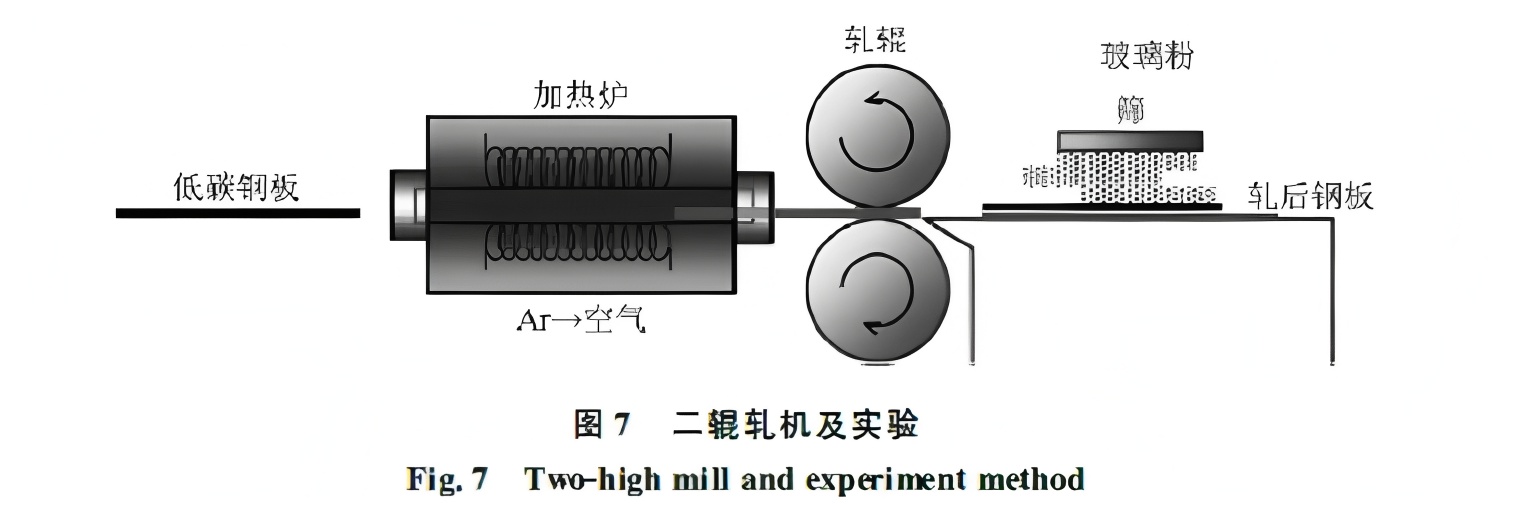
Yuan Kenichiro et al. used glass powder spraying to preserve scale and interface condition after rolling deformation. Using low-carbon steel rolled at 1000°C on a two-roll mill, with rapid glass powder application post-rolling (see Figure 7), they found that below 20% deformation, scale deforms uniformly. Above 30%, the scale fractures, and base metal extrudes through cracks, exposed to the surface, deteriorating quality. Thus, deformation should be limited to <30% at low rolling temperatures.
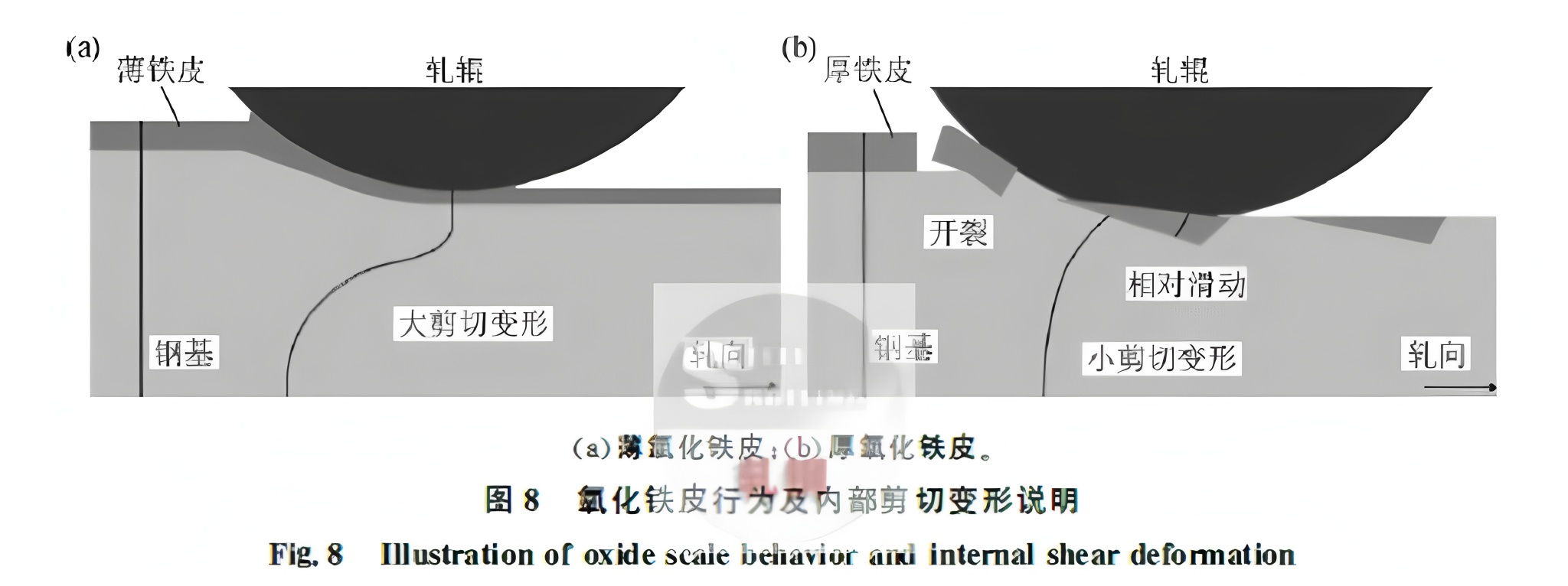
Further research showed that at 900°C and 40s oxidation, the scale indents into the matrix with a rough interface. At 1000°C, the scale cracks and base metal extrudes. At 1100°C, cracks form through the scale thickness, but the scale-matrix interface remains smooth. Thick scale introduces relative slip between rolls and matrix, making the scale prone to fracture, reducing shear deformation, and lowering rolling load (Figure 8). Longer oxidation time before rolling increases scale thickness, reduces ductility, and raises the likelihood of irregular deformation.
1.2.3 Effect of Mold Flux Adhesion on Red Scale Defects
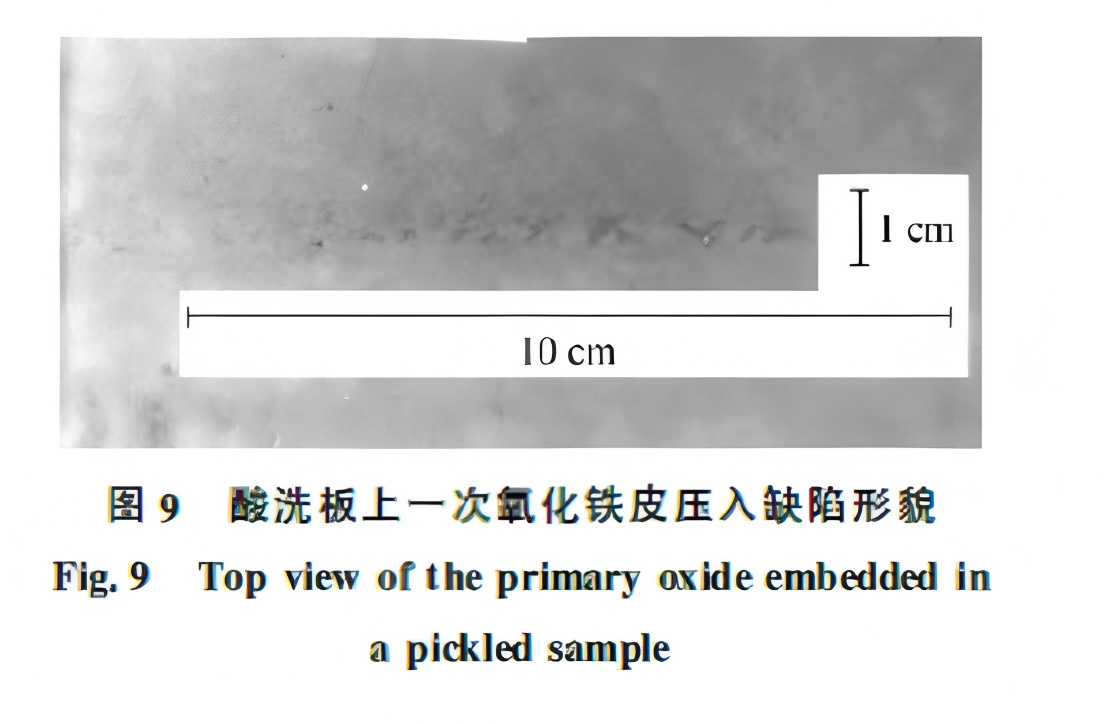
Jorge RAMIREZ-CUELLAR et al. studied the formation of primary oxide defects in CSP lines. The combination of time and temperature when the slab enters the furnace is decisive for primary scale formation. Slab vibration or stationary rolls increase the risk. Once pressed into the surface, scale can evolve into red scale defects during rolling. Figure 9 shows a “boat-shaped” primary scale indentation defect after pickling.
This study also linked mold flux to primary scale defects, finding exogenous elements like Si and Ca in primary scale, which enhance adhesion. Sprinkling mold powder at the furnace entrance, while not fully replicating the defect, confirmed that Si and Ca originate from mold flux.
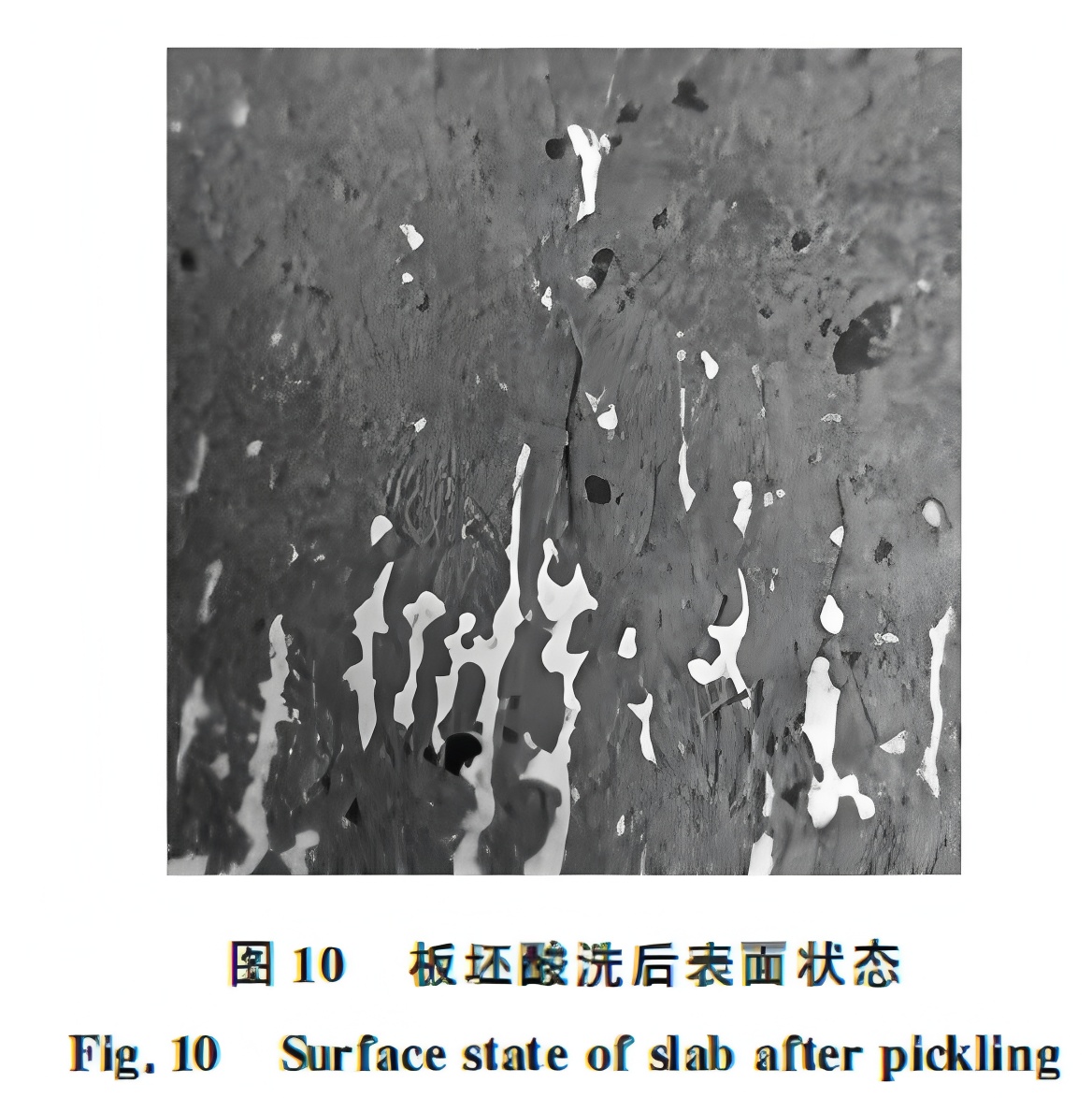
Mold flux adhesion is common. In conventional continuous casting, pickling reveals white residues in oscillation marks, which are mold flux (Figure 10). While this flux increases the risk of incomplete descaling, practice shows that most adhered flux is removed with the primary scale and seldom causes indentation defects in conventional hot rolling lines.
1.3 Questions Regarding Red Scale Formation Mechanism and Defect Control
1.3.1 Questions on the Formation Mechanism
The accepted theory attributes red scale to difficult-to-remove primary scale formed in the furnace due to silicon, which evolves during rolling. However, some observations are difficult to explain.
Using DP590 steel (0.07%C-0.45%Si), a sample was placed on a production slab and heated in a furnace (1220°C, 200 min, air-fuel ratio λ=1.05–1.30). The sample was then retrieved from the slab surface (Figure 11) and the scale-matrix interface examined after water quenching.

Figure 12 shows the interface morphology of the reheated sample. A network structure from liquefied Fe₂SiO₄ is evident (Fig. 12(a)), with an anchor structure wedged into the matrix (Fig. 12(b)), consistent with lab simulations (Fig. 3). Notably, a layer of fine oxide particles, 15–25 μm thick, exists on the matrix surface.
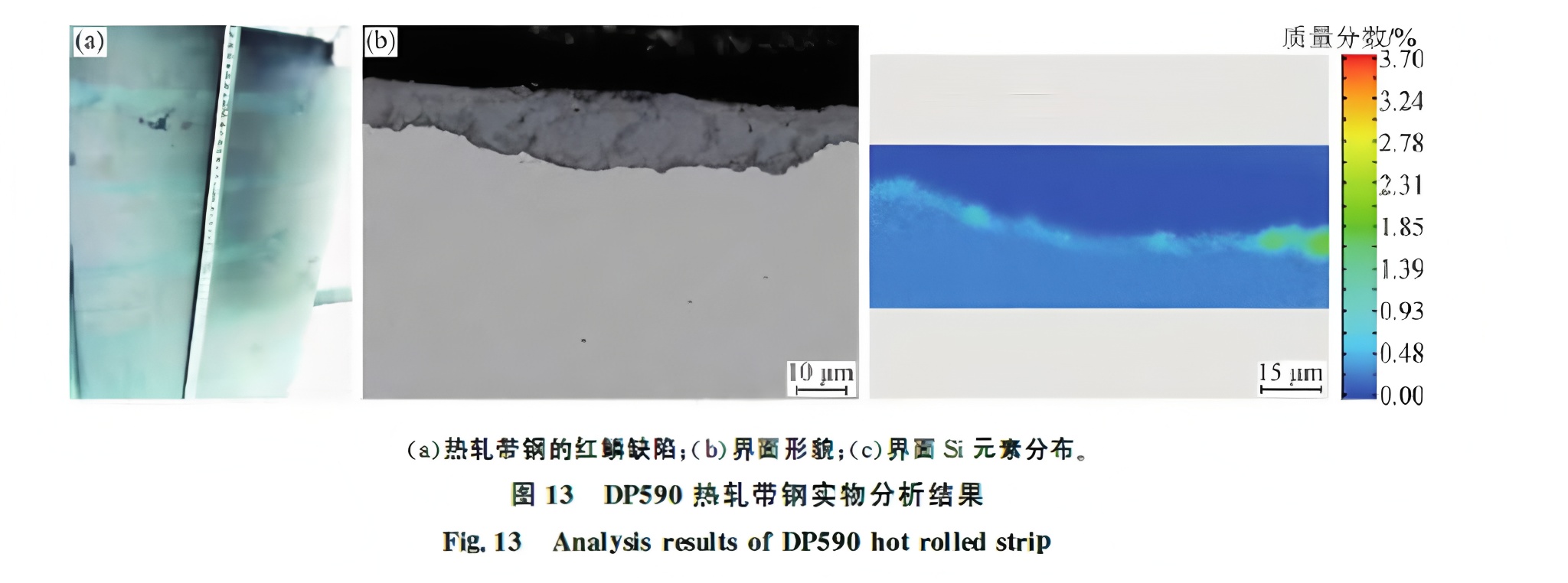
Figure 13 shows analysis of hot-rolled DP590 strip. Red scale defects are widespread, and silicon enrichment is found at the scale-matrix interface. Both red scale and normal areas show a Si-rich layer, but the scale is thicker in red scale areas. Comparing Fig. 13(b) with Fig. 12(b), the anchor structure and inner oxide particle layer are absent.
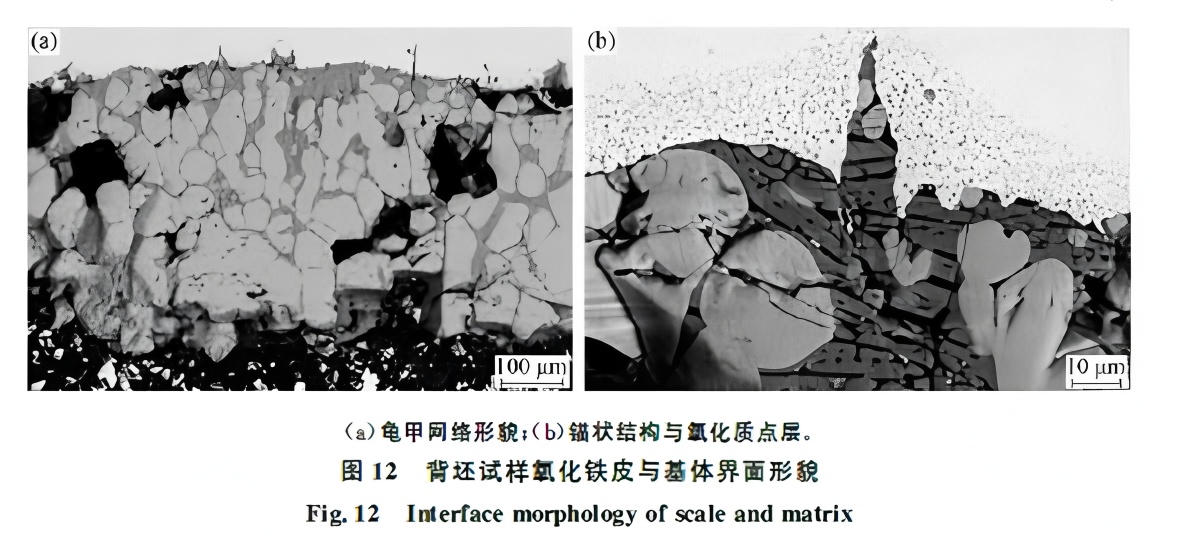
Hot rough rolling involves repeated oxidation and descaling. After furnace extraction, the slab undergoes initial descaling, followed by multiple passes of rough descaling during rolling, effectively removing the inner oxide layer. Since the oxidized particle layer in Fig. 12(b) is deeper than the anchor structure, its removal would also eliminate the anchor. Thus, it is questionable whether red scale defects in the final product originate from the FeO/Fe₂SiO₄ structure formed in the furnace (as in Fig. 2).
1.3.2 Control of Red Scale Defects – Banded Scale in Si-Containing Steel
Chun-Chao Shih et al. proposed a mechanism for banded scale formation in steel with ~0.2% Si. Using steels with 0.20% and 0.02% Si, with inter-stand cooling water between the first and second stands set at 5%, 10%, and 15% flow, banded scale was absent at 5%, slight at 10%, and clearly visible at 15%, with spacing matching that of the descaling nozzles.
Cross-sectional analysis is shown in Figure 14. Normal scale is ~4.8 μm thick with a smooth surface (Fig. 14(a)). Slightly banded black scale averages 8.2 μm thick, with some areas crushed (white dashed box) and others intact; the scale-matrix interface is undulating (Fig. 14(b)). In severe banding, the scale is completely fragmented into sub-2 μm particles, with areas of spallation. Scale thickness exceeds 11 μm, even 20 μm in places, with a highly distorted interface (Fig. 14(c)). All scales comprise Fe₃O₄, FeO, and α-Fe, without Fe₂O₃.
TEM shows Fe₂SiO₄ at the scale-matrix interface. Mn is dissolved in FeO without interfacial enrichment.
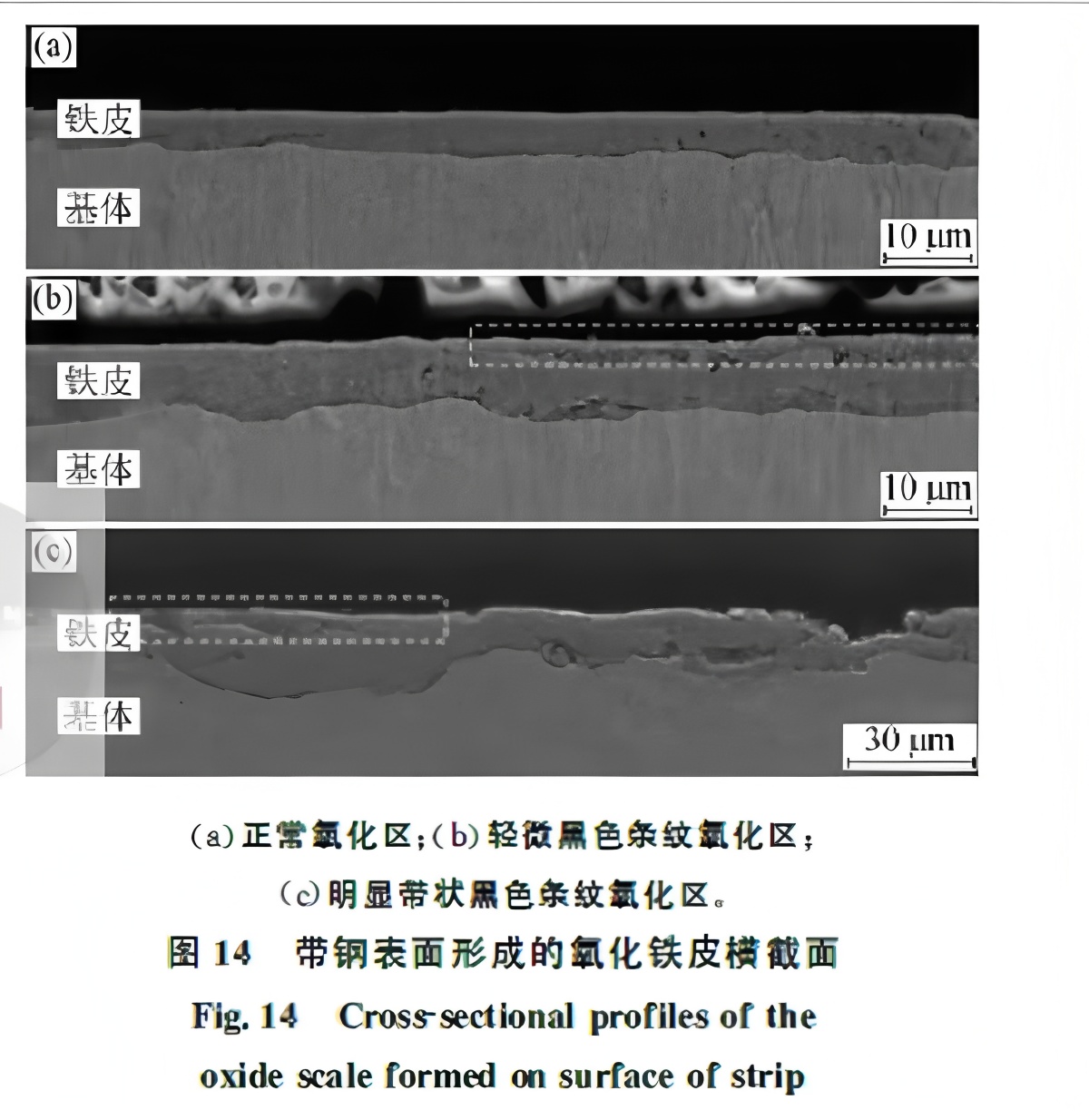
Notably, the severely banded scale in Fig. 14(c) appears black, but this does not preclude it from being a red scale defect. α-Fe₂O₃ powder under 2 μm appears red. Fragmented scale oxidizes rapidly to Fe₂O₃, the key element of red scale. The scale in Fig. 14(c) is fully fragmented into sub-2 μm particles, ripe for oxidation. Under experimental conditions, insufficient time may have allowed only Fe₃O₄ formation. Studies indicate that over 15% powder content is needed for visible reddening. In production, strip with 0.45% Si appears dark red in banded areas; thus, redness also depends on silicon content.
Descaling nozzle overlap causes localized overcooling, with temperature drops of tens of degrees (Fig. 15(a)). Theoretically, thinner scale should form in cooler regions. However, Chun-Chao Shih et al. observed the opposite, due to interactions between scale and mill components. As the strip enters finishing stands, contact with work rolls and inter-stand cooling further reduces surface temperature, possibly by 100–200°C. Heat transfer from the base metal can recover surface temperature. Figures 15(f)–(h) show that 0.02% Si steel forms uniform scale. For Si-containing steel, surface compounds greatly affect heat transfer. Silicon’s strong oxygen affinity leads to Fe₂SiO₄ formation at the interface, which has only one-third the thermal conductivity of FeO, hindering heat transfer.
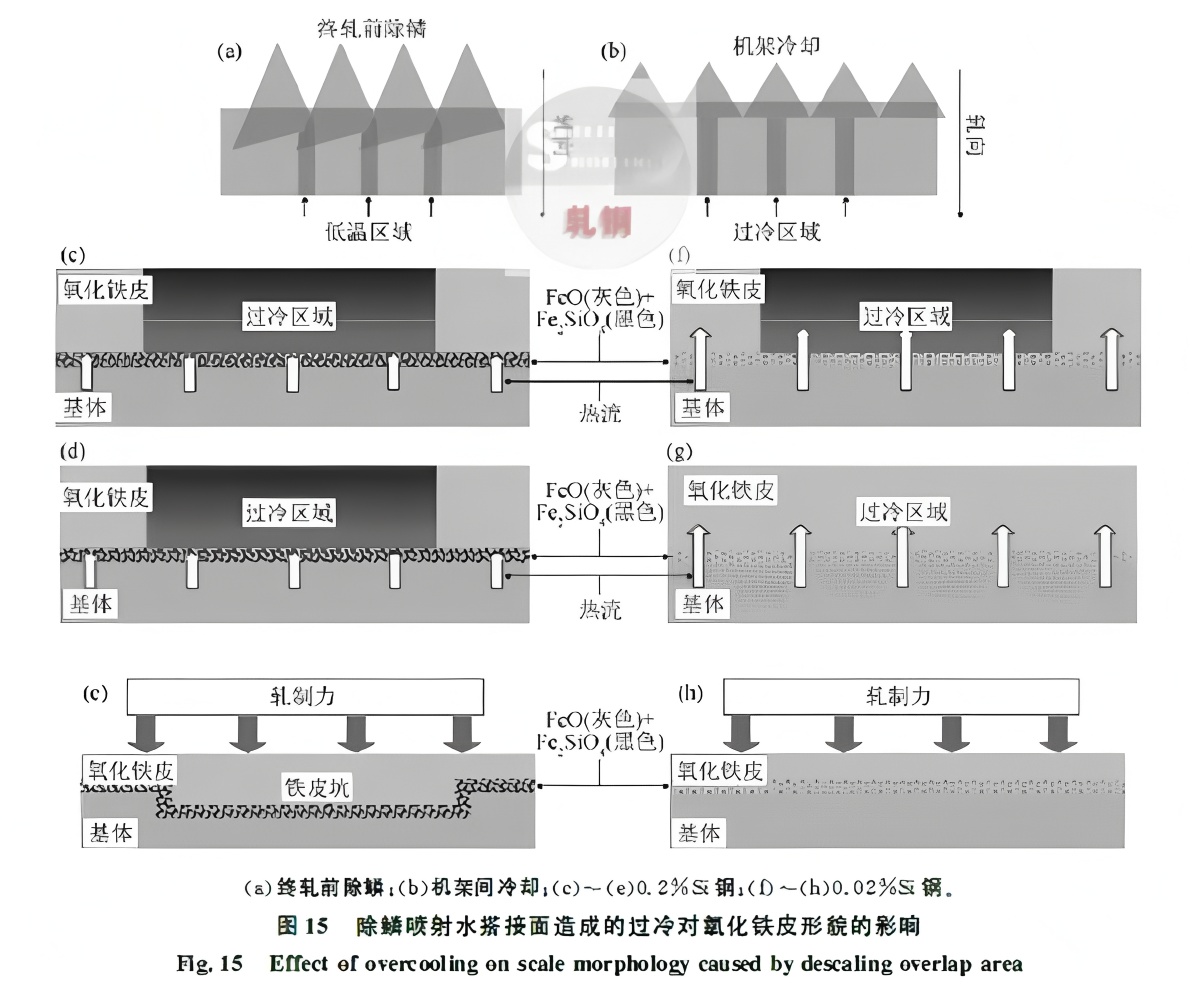
During finishing, the scale is mainly newly formed FeO, which deforms plastically above 700°C but steadily only above 1000°C. Finishing usually starts at 1000–1050°C. High mill cooling flow can reduce descaling overlap zone temperatures to as low as 700°C. Fe₂SiO₄ impedes heat recovery from the matrix, so the cooled scale may not regain temperature before subsequent rolling. As temperature drops, FeO’s deformability decreases. Under rolling force, low-deformability scale in cool zones elongates less than adjacent areas, is pressed into the matrix forming pits, and leads to variable scale thickness. With high cooling flow, numerous scale pits spread along the rolling direction, forming black bands (Figs. 15(c)–(e)) spaced at the nozzle interval. Low temperature makes the scale brittle; rolling and friction cause fragmentation, leading to widespread red scale defects in steels like DP590.
This process also suggests that primary scale from the heating furnace is not the sole cause of red scale defects.

